Driving clarity in clinical development
Powered by digital twins, data, and AI
Replace siloed workflows and black-box predictions with a unified, upstream workspace for trial design, planning, and analysis—so your teams reach alignment faster, test assumptions earlier, and move forward with confidence.
Real-world Results
Accelerating Clinical Development in Alzheimer’s Disease
We work in neuroscience, immunology, metabolic disease, and more.
Solutions
Confident decisions across the clinical development lifecycle.
One workspace for upstream trial design
Unlearn brings together the critical components required for trial design — where decisions are iterative, assumptions evolve, and rationale must remain clear and defensible across review cycles.
Continuously search, structure, and summarize relevant literature and regulatory precedent from sources like PubMed, ClinicalTrials.gov, and drugs@FDA — all in one place. Eliminate scattered searches and align on precedent in days, not weeks.
Explore harmonized clinical trial and real-world datasets to validate clinical and statistical assumptions. Assess population characteristics, endpoint behavior, and benchmarks.
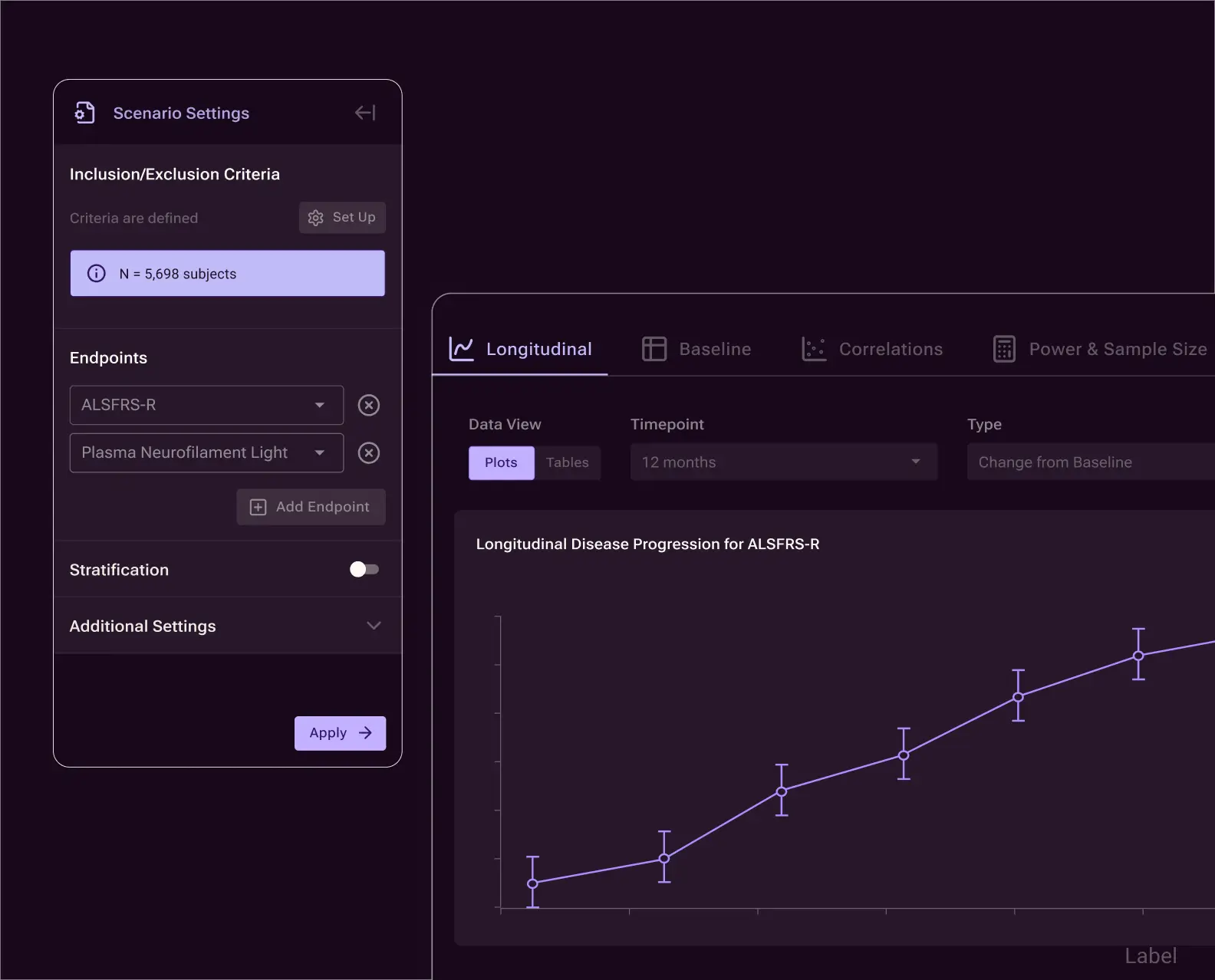
Build and compare trial-design scenarios to evaluate endpoints, inclusion/exclusion criteria, sample size, and constraints. Every scenario is reproducible, and linked to underlying evidence — supporting informed design trade-offs before protocol finalization.
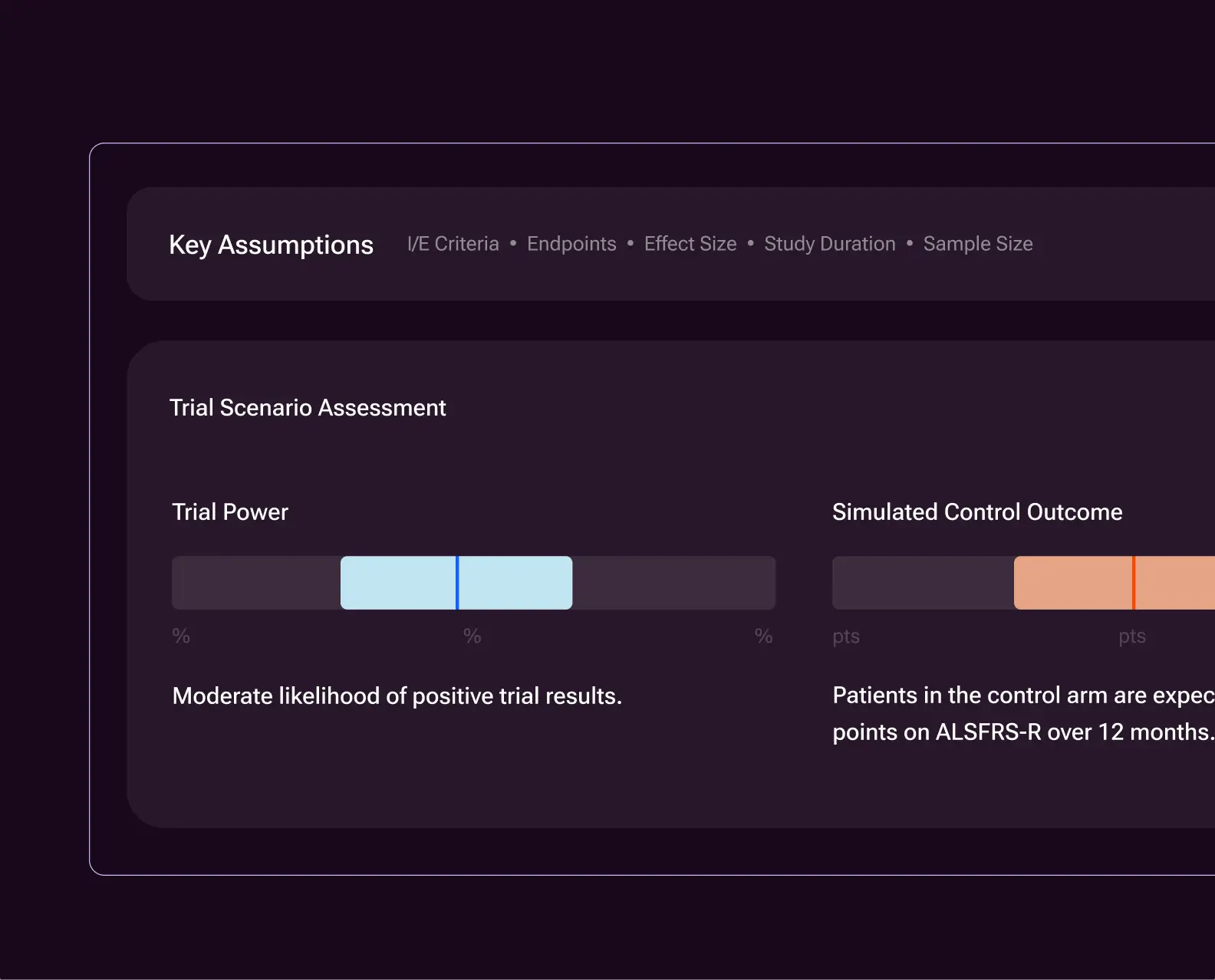
Strengthen trial analyses with digital twins—AI-generated forecasts of clinical trial participants’ expected control outcomes.
Used as external comparators in early-stage and open-label studies, digital twins reduce variability and improve the ability to detect treatment effects. The same approach extends to randomized trials, where digital twins can support smaller sample sizes or increased power.
This methodology is qualified by the EMA and aligned with current FDA guidance, enabling clearer go/no-go decisions earlier in development and more efficient late-stage trials, with measurable reductions in trial size, cost, and time to readout.
.webp)
.webp)
.webp)
Trusted by leading sponsors
Partnerships
Collaborating with leaders to move the industry forward.
Explore how leading biopharma companies are applying Unlearn’s AI-powered solutions in active studies today to reach clinical milestones faster.

Press

Press

Press
.png)




