Design clinical trials with speed and rigor
Reach confident, evidence-backed trial design decisions earlier — before protocols are finalized.
Unlearn’s TrialPioneer helps clinical development teams reach design decisions faster by replacing fragmented searches, spreadsheets, and one-off analyses with a single, unified AI-enabled workspace. Teams can investigate and compare trial design scenarios earlier, anchor assumptions to credible evidence, and enter governance discussions with clear, defensible rationale.
.svg)
How It Works
See how teams design, compare, and align on trial planning decisions earlier
- 01Explore trial precedent, observed data, and simulations in a single environment
- 02Compare endpoints, eligibility criteria, and design scenarios without building custom analyses
- 03See how protocol choices change populations and expected outcomes
- 04Iterate on design decisions live and understand trade-offs in real time
- 05Preserve assumptions, evidence, and results so rationale doesn’t disappear after meetings
Product Overview
An AI-enabled system for evidence, assumptions, and trial design scenarios
TrialPioneer brings together the critical components required for early planning and design— where decisions are iterative, assumptions evolve, and rationale must remain clear and defensible across review cycles. Clinical development teams work from a shared source of truth, reducing rework and accelerating alignment.
AI-powered literature and precedent review
Seamlessly search, structure, and summarize relevant scientific and regulatory precedent from sources such as PubMed, ClinicalTrials.gov, and drugs@FDA. Scout replaces scattered searches with a transparent, shared foundation for evidence review — helping teams align on precedent in days, not weeks.
Historical data exploration
Explore harmonized clinical trial and real-world datasets to validate clinical and statistical assumptions. Assess population characteristics, endpoint behavior, and benchmarks using relevant historical data — grounding early design decisions in evidence rather than intuition.
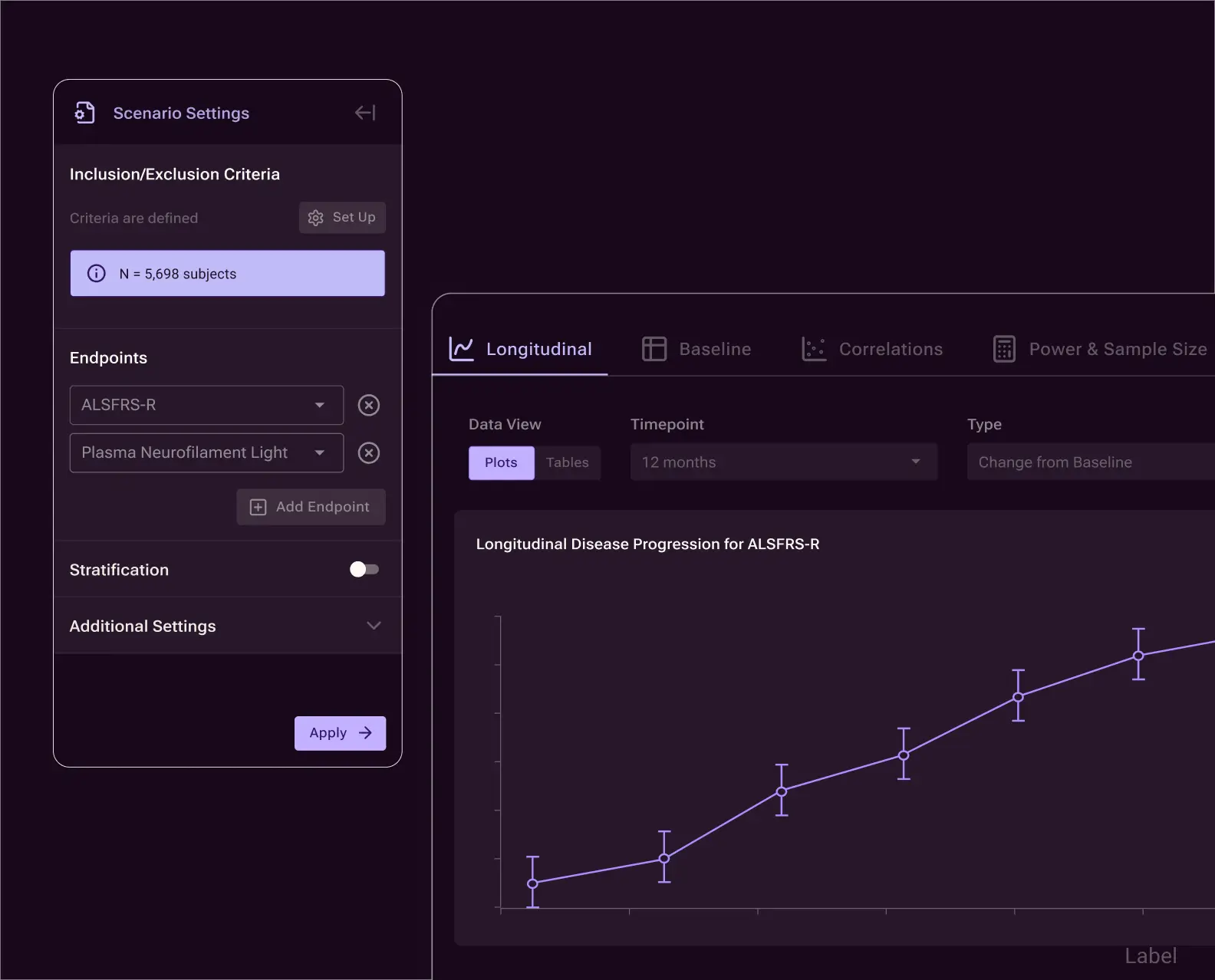
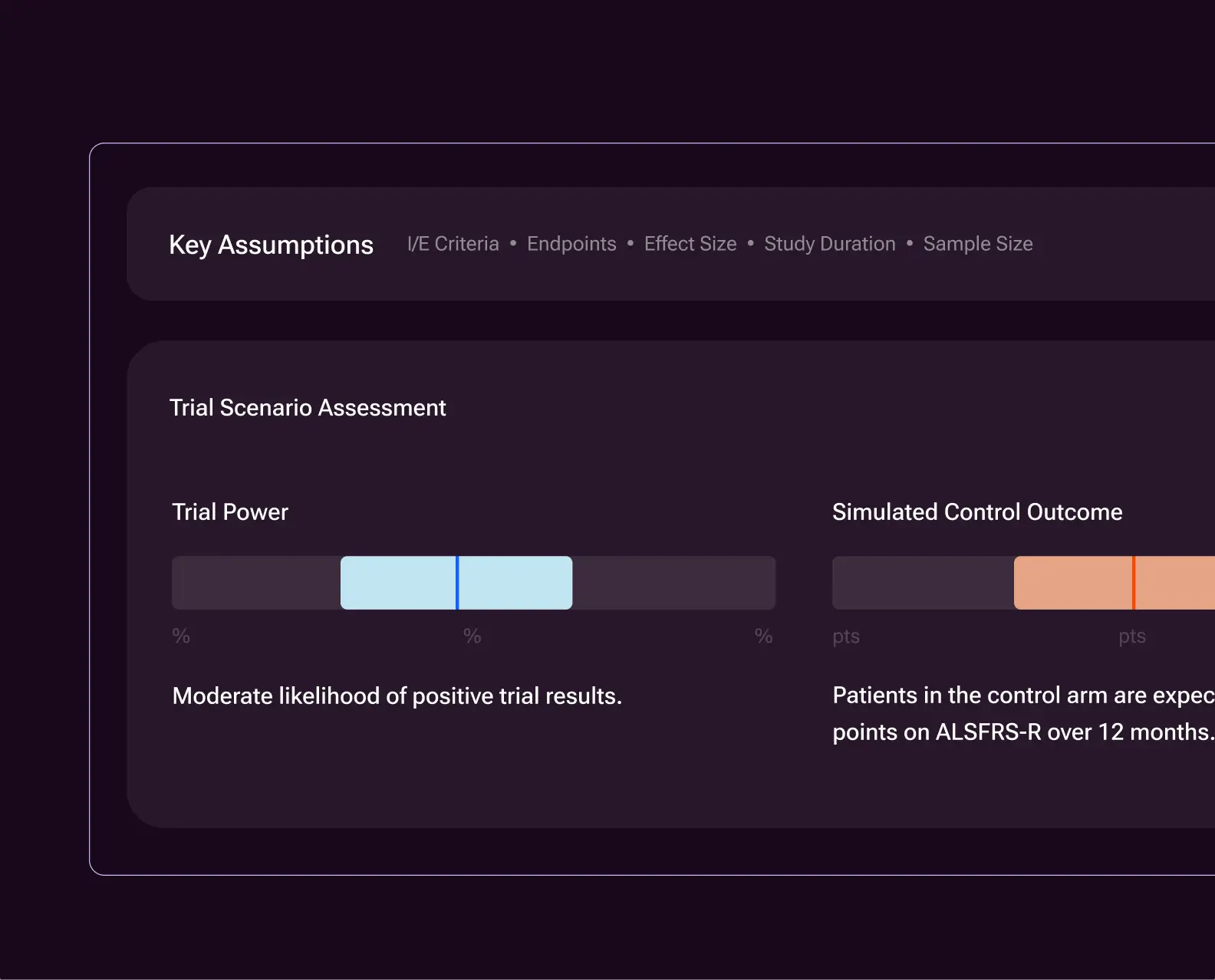
Explainable trial simulations
Automatically link, build, and compare trial design scenarios across endpoints, eligibility criteria, and sample size. Every scenario is explainable, reproducible, and grounded in historical evidence — supporting informed trade-offs before protocol finalization.
.webp)
Benefits
Faster design cycles without sacrificing scientific rigor
.jpg)
Real-world Impact
TrialPioneer
in action
Optimize study design, including endpoint, time point and eligibility strategies
How We're Different
Built for trial planning — and scientific oversight
Unlearn supports evidence review, assumption testing, and scenario comparison before designs are finalized.
TrialPioneer makes assumptions, inputs, and outputs visible across clinical, biostatistics, and regulatory teams earlier.
Unlearn links evidence, historical data, and simulations into a transparent, review-ready workflow.
TrialPioneer focuses upstream, where early design decisions matter most.





